It's this one: Highly Efficient and Selective Dissolution Separation of Fission Products by an Ionic Liquid [Hbet][Tf2N]: A New Approach to Spent Nuclear Fuel Recycling Fang-Li Fan, Zhi Qin, Shi-Wei Cao, Cun-Min Tan, Qing-Gang Huang, De-Sheng Chen, Jie-Ru Wang, Xiao-Jie Yin, Chao Xu, and Xiao-Gui Feng Inorganic Chemistry 2019 58 (1), 603-609.
They give a nice prep of the ionic liquid which is a simple metathesis reaction, they mix the lithium salt of the imide and the chloride salt of betaine, and the ionic liquid oils out, can be washed with water and made chloride free. I am always interested ionic liquids that are insoluble in water, so I'm making a mental note of this one.
One difference between the extraction used in the paper cited in the OP and the procedure utilized in this paper, besides the temperature (400K, 127°C) as noted by the authors of the Chem. Commun. paper, is that in this case, the ionic liquid is saturated with water.
The authors of the Inorg. Chem. paper just cited have a nice table of solubility for the Ln and An species:
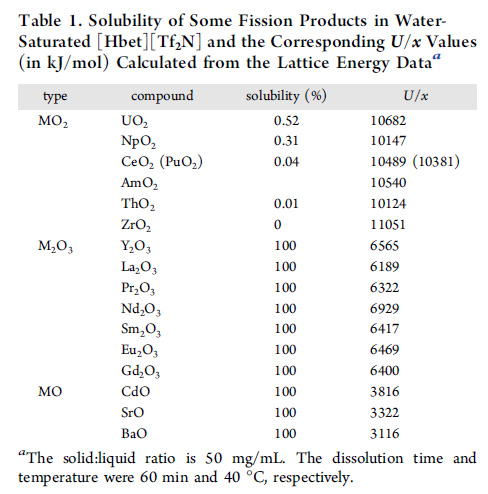
Under these conditions, the water saturated ionic liquid at 40°C, uranium and neptunium oxides are only sparingly soluble, americium not at all, but all of the lanthanides are very soluble. It would be interesting to see how the solubility changes with desiccation by heating and with heat itself. It suggests a path toward continuous crystallization if both papers are accurate, which conceivably as the authors of the Chem. Commun. paper suggest.
I should check out some of the other references in the Chem. Commun. paper, particularly references 17-21, the majority of which are Binnemanns' papers. Binnemanns is a "go to" guy for ionic liquids and actinides.
Betaine, of course, is very cheap and readily available. I don't know much about it's radiation stability, but it's a simple enough molecule that it should be easy to remove any radiolysis products. I know I have a bunch of papers in my files from James Wishart at BNL on the radiation stability of ionic liquids, but I haven't looked at them in a number of years. I'm sure he must have commented on the radiation stability of Tf2N anions. Probably the degradation products are simply triflamides.
These are both nice papers. I like them a lot.
