Science
Related: About this forumOn Being Old and Naive: Studying Concepts for Discussions with My Son at Thanksgiving, Intermetallic Solubility.
My son is working on 3D printed metallic alloys for nuclear applications for his Ph.D.. It has been my habit to prepare whenever I meet someone more knowledgeable than I am on a particular topic, say a scientist famous for his, her or their work, in a particular area, I do a brief literature survey to update my knowledge on that topic so that I can converse with him, her or them, if on a superficial level. My son certainly isn't famous in his topic, although his advisor is, but he certainly knows more than I do, and you never know, he may be famous within his narrow field some day, should science survive in the United States, or come back on some level in a post-Fascist American era.
For several years I have been interested in the properties of liquid plutonium, which as it happens, is a very good solvent for other metals, and thus have been studying peritectic, eutectic, and eutectoid reactions involving plutonium as a pathway to a certain type of "breed and burn" nuclear reactors which may be designed to run at full power for decades using depleted or once-through uranium from used nuclear fuels. Much of the literature concerned with these concepts dates from the 1950's and 1960's before the tragic rise of antinuclear ignorance, coupled with the development (shortly before antinuclear ignorance prevailed) of a nuclear power industry centered around a single concept, largely light water reactors with some heavy water reactors but overwhelming, with minor exceptions, on a thermal neutron spectra. As a result, creativity in nuclear power concepts waned.
However a paper actually published in this century related to plutonium alloys came into my purview some years back, this one:
D E Dooley et al Development of an electronic phase diagram and the predictions of plutonium alloy phase stability using electronic properties , J. Phys.: Condens. Matter 13 8677 2001, which actually provides a Gurry-Darken Plot and beyond for plutonium alloys but my self-impression is that I didn't pay much attention to the and beyond at the time I accessed it, being only interested in liquid plutonium.
Anyway, a paper was published recently informed me that I am stuck thinking in an old way, that metal phase relations are advanced well beyond my somewhat primitive conceptions.
To wit, I encountered this paper today:
Beyond Hume-Rothery Rules, Andrew Martin and Martin Thuo, Accounts of Materials Research 2023 4 (10), 809-813.
From the introductory text:
In creating these semi-empirical rules, no reference to size of the material is made, even though solid solutions are needed from the nano- to the macroscale. In many metal processing techniques, especially with recent advances in additive manufacturing, metallic powders are required. How will these rules apply with associated surface-to-bulk constraints at ambient conditions? Surface oxides and curvature-dictated pressure jump conditions must be considered. Herein, we revisit solubility/mixing in metals, particularly considering surface oxidation and the associated speciation. Fundamentally, because all of these rules have not utilized broader thermodynamics considerations (PV (π ) (pi)and non-PV (τ )(tau) work, see Figure 1), there is a need for a different viewpoint...
...Thermodynamics, however, is a tool that allows us to determine what is likely to happen or not. In a simplified approach, the evolution in ΔGmix (del Gmix) is often evaluated against stoichiometry (Figure 2a). Considering kinetics, we realize that the mixing materials are in different phases at some point, which should in turn perturb free diffusion and affect the overall free energy due to the generation of new interfaces (Figure 2b). (22) Extending the existence of phases to include surfaces/interfaces work (ΔGmix,τ ) (Del) (tau) ( leads to a complex free energy space (Figure 2c) that is akin to one we highlighted before. Given that surfaces evolve with their surroundings, such an energy landscape is dynamic and an easier entry point to engineering the entire materials state. The surroundings-dictated non-PV work (τ ) (tau) affects not only the kinetics of a reaction (diffusion) but also its thermodynamic state (Figure 2b). Although including this dimension in the free energy landscape adds complexity, it represents a more accurate depiction of miscibility in a real system, especially in powders...
(Note: In the text I spelled out Greek letters in parentheses to get around the limits of the DU editor)
Some graphics from the paper:
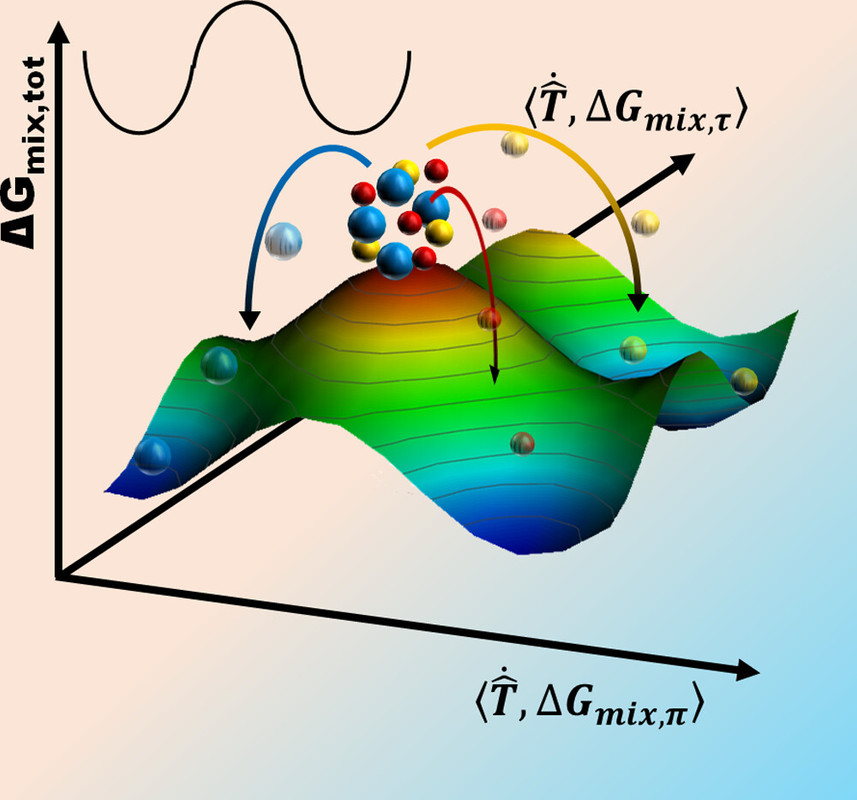
The caption:
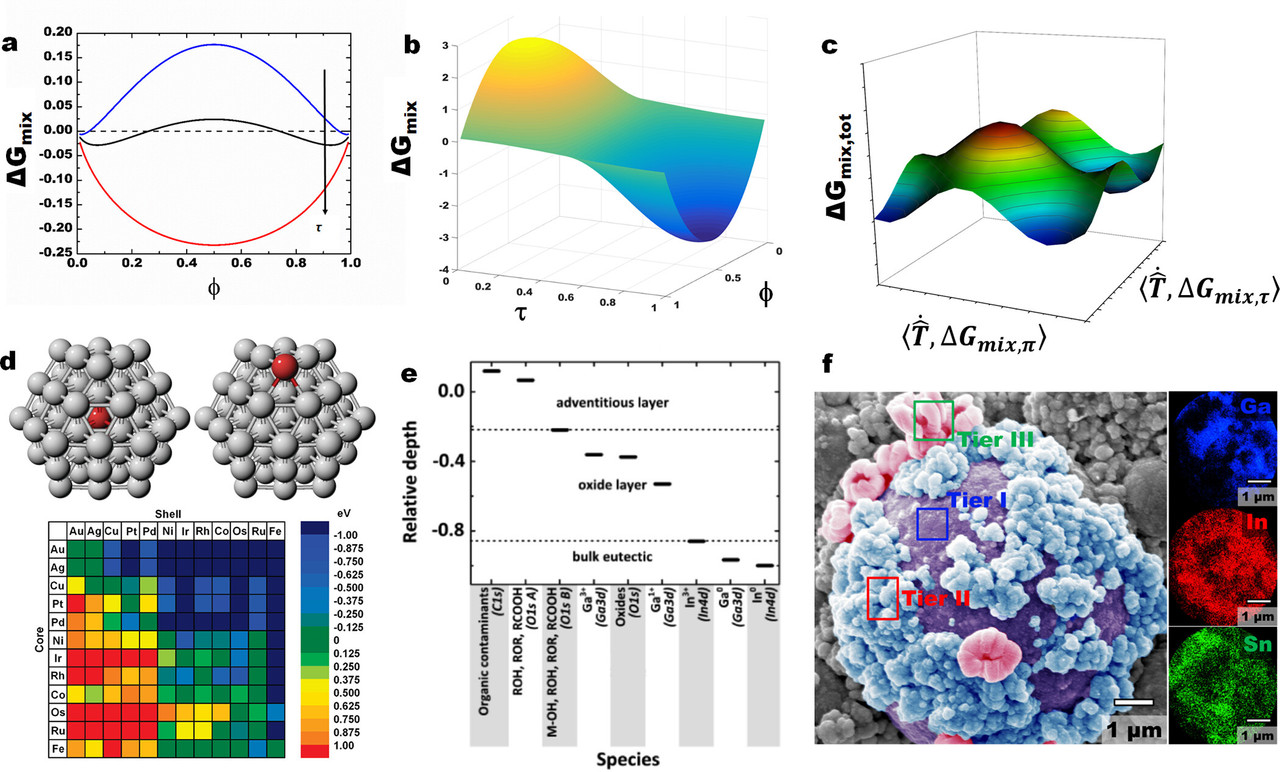
The caption:
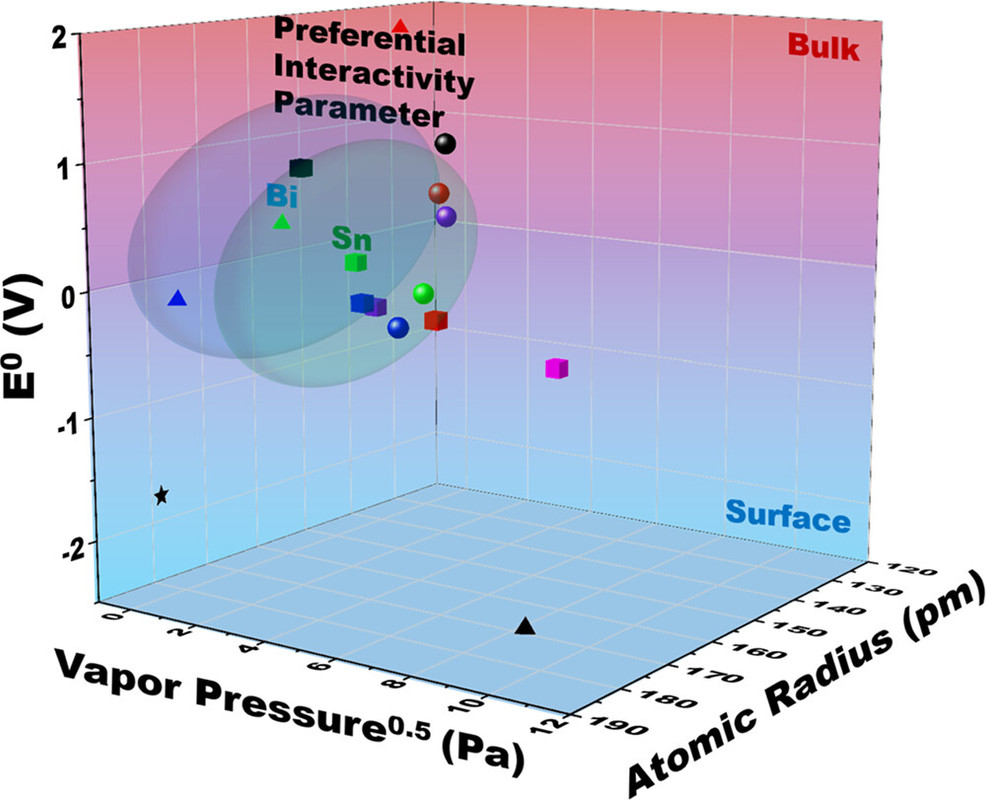
The caption:
I have never discussed this particular issue with my son, although when he was selecting a college in his junior year in high school, we had a nice discussion with graduate students and faculty members in the Materials Science Department on high entropy alloys at the University he ended up attending. (Figure 1 suggests some issues that may appear in high entropy alloys at high temperatures.)
We'll see if I can hold my own with my son, and/or I end up with an opportunity to be smug in discussing issues he hasn't put in his intellectual portfolio yet. (Probably wishful thinking on my part.)
My son is smarter than I am, has more intellectual raw power, but perhaps not quite as well educated (yet) as I am. As I told some of my colleagues at work, it's not that I'm particularly smart; it's just that I've lived a long time.
I trust you will enjoy the last Thanksgiving in the dying era of the US Democracy.
erronis
(22,688 posts)I agree that pure and even applied science may be under threat soon.
ThoughtCriminal
(14,706 posts)It always ended badly when one of my great aunts would start citing research that had not been published or even peer reviewed.
So we will stick to football this holiday season.
NNadir
(37,313 posts)My favorite read simply, "Facts are facts."
I wore it to my dermatologist appointment, and my doctor, remarked how much he liked it, and I said, "It's a tautology that shouldn't be controversial but somehow has become so."
(This year the AAAS is sending laptop computer sleeves - sigh.)
Discussing the facts about thermodynamics here and elsewhere often gets into spaces implying that its laws are subject to change by wishful thinking.
I could certainly believe the topic could be controversial among some people. You hear all the time descriptions of what are effectively perpetual motion machines, or claims, even worse, that storing energy is a good idea. It isn't.
I certainly want to scream when I hear treatments of stored energy as if it were primary energy.
littlemissmartypants
(31,678 posts)Congratulations on your DU milestone!
Living a long time can have its perks. I hope you're around much, much longer for more of them.
![]()
❤️